

  |
GLIWICE RADIOCARBON LABORATORYAMS DATINGThe development of the accelerator mass spectrometry (AMS) technique in the 1980's enabled 14C dating of samples containing as little as a few milligrams of carbon, which is ca. 1000 times less than in the conventional techniques. The relative numbers of the atoms of different carbon isotopes in the sample are directly measured and the radiocarbon age is determined. PREPARATION STEPSA system for the preparation of samples for AMS dating has been developed in the Gliwice Radiocarbon Laboratory in 1999. As yet, the system has been used to produce graphite targets from plant macrofossils, charcoal, peat, bones, shells and pollen extracts. Due to the very small sample amount, considerable effort is put into avoiding contamination with either modern or inactive carbon during the sample preparation. The purpose of the preparation of samples before radiocarbon measurement is the extraction of material that contains indigenous carbon in a quantity sufficient to measure the 14C content, remove contaminating substances which usually give different age, and produce the medium for appropriate measurement technique (e.g. CO2 for gas proportional counters or graphite for AMS). Chemical pretreatment
The procedure of treatment depends on the sample material. Usually the samples need physical cleaning or separation under microscope. Most commonly the AAA (acid-alkali-acid) method is used for organic samples like charcoal or organic remains. Shells are cleaned and the outer part is dissolved in a weak acid. For bones the collagen is extracted according to the modified Longin's method (see poster) CombustionAfter the chemical pretreatment the sample material in a quantity corresponding to ca. 1mg of carbon is placed into a quartz tube with copper dioxide (the source of oxygen needed for combustion) and silver wool (for the removal of gaseous sulphur and chlorine compounds). The tube is then kept for several hours at 900°C. 


Purification of CO2
The tube with the CO2 derived from the sample is incised and placed into an arm of a vacuum line with a ball joint. After overnight pumping to high vacuum (10-4 mbar) the arm is cut off with a cock and the tube is cracked and gas released. Water vapour is frozen in a trap cooled with a mixture of dry ice and alcohol (ca. -70°C) and the CO2 is collected in a sealed glass vial. The amount of obtained CO2 is measured and the excess of gas is removed or stored in a separate vial. Graphitisation
The CO2 is reduced to graphite during the reaction with hydrogen at the temperature of 600-630°C (dependent on the reduction rate for a given sample) in the presence of iron as a catalyst. The graphite is deposited on the iron powder introduced into a small quartz tube. The reactor and iron powder are previously heated overnight at 90°C under continuous pumping. Directly before the graphitisation the iron is oxidized and reduced in order to increase its catalytic properties. Then CO2 and H2 are introduced in stechiometric amounts, with ca. 20% excess of H2. During the reduction H2O is produced and continuously removed by freezing out in a mixture of dry ice and alcohol. The progress of the reaction is monitored by measuring the pressure. The reaction is usually complete after 3 to 4 hours. Pressing the graphite targetFe-C powder is pressed into a tablet which is used as a target in the sputter ion source of the accelerator. The target is stored in argon atmosphere until the measurement. 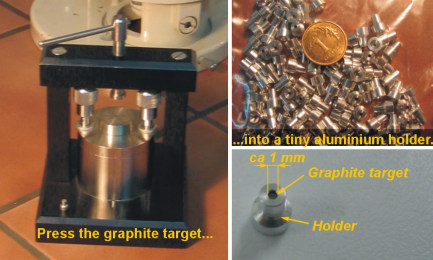
14C MEASUREMENTPrepared graphite targets are sent to an AMS laboratory for the measurement (at present to Poznań Radiocarbon Laboratory, Poland or to Leibniz Laboratory for Radiometric Dating and Isotope Research in Kiel, Germany). Each batch of samples is accompanied by at least two modern standard (Oxalic Acid) and two background (coal or marble containing no radioactive carbon) samples, prepared in the same way as samples of unknown age which are used for the age calculation. In the AMS technique, the graphite targets are loaded in the sputter ion source which produces a beam of negative carbon ions. Subsquently, low-energy mass analysis is performed with the use of magnets and sometimes also electrostatic analysers. The negative ions of the isotope of interest are accelerated to the terminal of the accelerator at a potential of at least 0.5 up to several million volts (MV). The negative ions are converted there to positive ions by the removal of several electrons during a stripping reaction with a gas (usually Ar) or carbon foils, and accelerated further to ground potential. After subsequent magnetic and electrostatic analysis, the ions are identified in an ion detector. The schematic layout of AMS spectrometer is presented below (adapted from Tuniz et al., 1998): 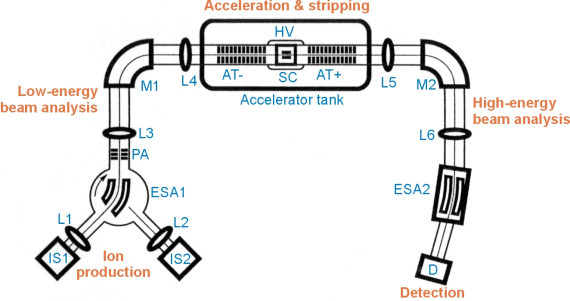
Abbreviations used on the figure: For more details about construction and performance of AMS spectrometers visit:Compact Carbon AMS at Poznań Radiocarbon Laboratory, Poland (also in Polish).Low energy AMS spectrometer (called Tandy) at ETH Zurich, Switzerland or their 6MV machine. You may also take the "AMS Instrument Tour" (2.5MV AMS spectrometer) presented by Woods Hole Oceanographic Institution, Massachusetts, USA. And you may well try some other links from www.radiocarbon.org. |